Quotes from Abstract
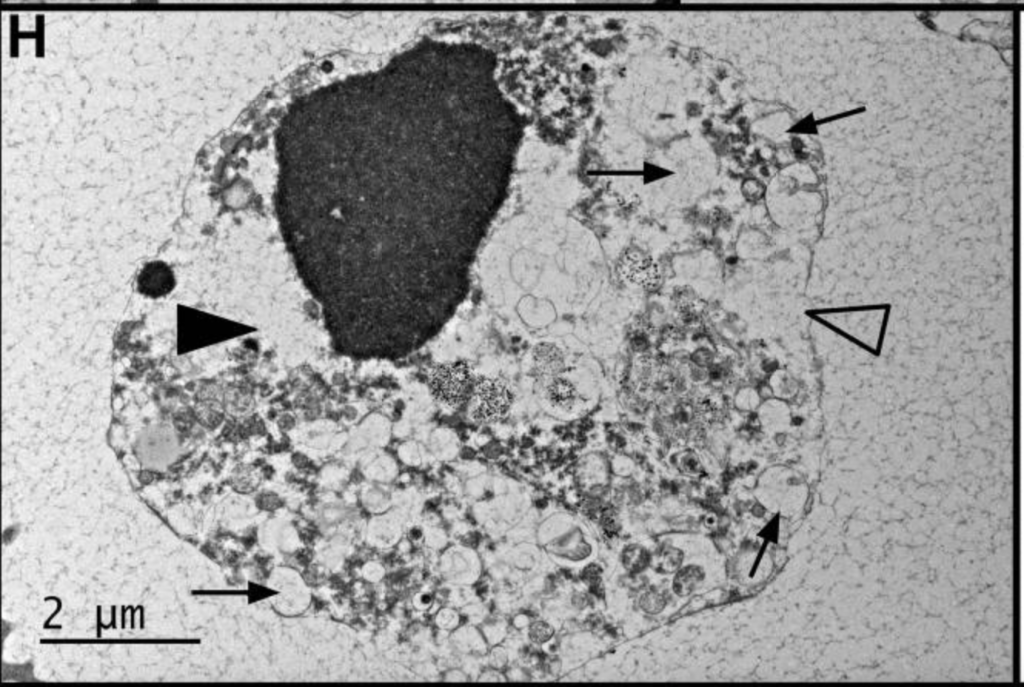
TEM analysis of CD3/CD28-stimulated T cells suggested a significant increase in the levels of apoptotic and necrotic cell death in T cells from ME/CFS patients (over 2-fold). Stimulated T Cells of ME/CFS patients also had higher numbers of swollen mitochondria.
We also found a large increase in intracellular giant lipid droplet-like organelles in the stimulated PBMCs from the extremely severe ME/CFS patient potentially indicative of a lipid storage disorder.
Lastly, we observed a slight increase in platelet aggregation in stimulated cells, suggestive of a possible role of platelet activity in ME/CFS pathophysiology and disease severity.
These results indicate extensive morphological alterations in the cellular and mitochondrial phenotypes of ME/CFS patients’ immune cells and suggest new insights into ME/CFS biology.
This is a small blinded study (4 participants) from with lead authors Fereshteh Jahanbani and Rajan D. Maynard [1] from the Stanford Center for Precision Medicine. The 2 patients were diagnosed used the Canadian Consensus Criteria [2], International Consensus Criteria [3] and SEID Criteria* [4]. (*This last definition is horrible I am including it for informational purposes only.)
One main takeaway is that this study provides additional confirmation for the mitochondria being an important, perhaps even central, locus of disease in myalgic encephalomyelitis. The findings of abnormal mitochondria serve as additional confirmation of several earlier studies which implicated mitochondria [5-11]. Additionally through the use of electron microscopy, Fereshteh Jahanbani et al. uncovered several cellular and subcellular abnormalities which could be potentially significant in the disease process, either as causes or as downstream effects several of which have also been found in earlier studies [6,7,12].
These abnormalities are:
- an increase in giant lipid droplet-like organelles in stimulated cells from patients,
- higher percentage and number of swollen mitochondria in patients’ stimulated T cells
- an increase in platelet aggregation in stimulated cells from patients,
- an over 2-fold increase in apoptotic and necrotic cell death in T cells from patients.
Apoptotic and Necrotic T Cells are increased in the ME patients

At 500-1500x magnification there was a 2.7 increase of necrotic stimulated T cells and 3.2 increase of apoptotic stimulated T cells [1]. Interestingly, the moderate patient’s T cells mostly had an increase in necrosis while the severe patient’s had an increase in both apoptosis and necrosis (apoptosis is programmed cell death while necrosis is unprogrammed cell death).
In the T-cell free PBMCs when unstimulated only a slight increase in necrosis was seen in the ME patients cells. When the PBMCs were stimulated a slight increase in necrosis was seen in the severe ME patient’s sample compared to the unrelated control [1].
Since the increased apoptosis has been found in several studies [6, 12, 13] two questions naturally arise: “what is the relation of the increased apoptosis to the disease mechanism?” and “could increased apoptosis be used as the long-elusive blood-based biomarker?”
While the answer to the first question seems to be unknown at present it the answer to the second questions seems to be, at least, “potentially.” Most other diseases don’t have their cells dying at a higher rate—at least not the cells in the blood. The increased apoptosis and necrosis in blood cells seems to me to unscore the systemic nature of this disease.
Giant Lipid Droplets
Stimulated PBMCs (peripheral blood mononuclear cells) contained lipid droplets and these droplets correlated with disease severity with the authors noting the percent of cells containing them in the severe patient (7.8%) was “remarkably higher” than in the matched healthy control (1.6%) [1]. I think it is very likely the lipid droplets are connected to the abnormal mitochondria and problems with energy production in this disease. Could it be that if the cells cannot produce enough energy compensatory mechanisms like production of lipid droplets might be upregulated much more than in healthy controls? Another explanation could be there that is a lipid product the body, for reasons unknown, cannot metabolize.
Swollen Mitochondria in Stimulated T-cells

Stimulated T-cells from the two myalgic encephalomyelitis patients had higher numbers of swollen mitochondria [1]. The difference was even more apparent when comparing the percent of cells with 3 or more swollen mitochondria (the cells had, on average, 8-9 mitochondria per cell). Twenty-five percent of patients’ stimulated T cells had 3 or more swollen mitochondria while only 5.3 percent of control stimulated T cells had 3 or more. In the words of the authors:
“These results suggest a positive correlation between disease severity and the extent of mitochondrial damage at single cell level after stimulation”
Platelet Ultrastructure by Electron Microscope
An increase of platelet clump and rosette-like platelet formations was seen in stimulated T-cells from ME patients but the increase was not significant, possibly due to the small sample size (2 patient, 2 controls). An increase in platelet clumps and large platelets was significant when only the severe patient was compared to the matched healthy control, possibly indicating platelet hyperactivation is associated with disease severity. Especially interestingly, these results seem to be in line with the just-published study of Massimo Nunes, Amy Proal et al which found microclots in the blood ME patients (and earlier long-COVID) at a higher rate than healthy controls [14].
Exome Sequencing
The sequencing of exomes (the parts of a gene which remain after RNA splicing) of patients and controls revealed the severe ME patient had a rare homozygous sphingomyelin phosphodiesterase 1 (SMPD1) variant of uncertain significance [reference needed]. The authors thought this noteworthy because mutations in SMPD1 are associated with Niemann Pick Disease type A/B: a lipid storage disorder [1]. The authors hypothesize the mutation in SMPD1 could be responsible for the increase in lipid droplets seen in the severe patient [1].
(Though the following is only my own speculation, I suppose if the SMPD1 gene variant was responsible it could occur like this: a severe acute infection leads to a high presence of sphingomyelin substrates, the mutated enzyme is unable to break them down effectively, and the continuing inflammation produces a new steady-state which again has too-high sphingomyelin for the enzyme to remove. If I remember correctly, some inborn errors of metabolism present in this manner, with an acute stress causing the body to suddenly lose the ability to compensate for the altered metabolite; though usually in childhood or adolescence.)
Summary and Thoughts
While this study is very small the deep investigation of a couple patients allows for discoveries which might not be made in a study investigating more patients but in a shallower scope (such as level of cytokines, or mRNA). Two findings that leapt out at me were the findings of abnormal mitochondria and the increased cell death (apoptosis and necrosis). Both the findings of abnormal metabolism, implicating the mitochondria, and increased apoptosis have been found in earlier studies, sometimes the same one [6, 8, 12, 15]. It may be that these two findings, abnormalities of the energy production in the mitochondria and increased cell death will be found to be characteristic of the disease and/or related to the disease mechanism. Many of the other findings (increased lipid droplets, increased platelet clumps, abnormal lipid processing-gene) also seem to be things which could fit with a model of altered metabolism and clearly need further investigation.
References:
- Jahanbani F, Maynard RD, Sing JC, Jahanbani S, Perrino JJ, Spacek DV, Davis RW, Snyder MP. Phenotypic characteristics of peripheral immune cells of Myalgic encephalomyelitis/chronic fatigue syndrome via transmission electron microscopy: A pilot study. PLoS One. 2022 Aug 9;17(8):e0272703. doi: 10.1371/journal.pone.0272703. PMID: 35943990; PMCID: PMC9362953.
- Carruthers B.M., Jain A.K., De Meirleir K.L., Peterson D.L., Klimas N.G., Lerner A.M., Bested A.C., Flor-Henry P., Joshi P., Powles A.C., et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Clinical Working Case Definition, Diagnostic and Treatment Protocols. J. Chronic Fatigue Syndr. 2003;11:7–36. doi: 10.1300/J092v11n01_02.
- Carruthers BM, van de Sande MI, Meirleir KLD, Klimas NG, Broderick G, Mitchell T, et al. Myalgic encephalomyelitis: International Consensus Criteria. J Intern Med. 2011;270: 327–338. doi: 10.1111/j.1365-2796.2011.02428.x [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. Washington (DC): National Academies Press (US); 2015 Feb 10. PMID: 25695122.
- Hoel F, Hoel A, Pettersen IK, Rekeland IG, Risa K, Alme K, Sørland K, Fosså A, Lien K, Herder I, Thürmer HL, Gotaas ME, Schäfer C, Berge RK, Sommerfelt K, Marti HP, Dahl O, Mella O, Fluge Ø, Tronstad KJ. A map of metabolic phenotypes in patients with myalgic encephalomyelitis/chronic fatigue syndrome. JCI Insight. 2021 Aug 23;6(16):e149217. doi: 10.1172/jci.insight.149217. PMID: 34423789; PMCID: PMC8409979.
- Missailidis D, Annesley SJ, Allan CY, Sanislav O, Lidbury BA, Lewis DP, Fisher PR. An Isolated Complex V Inefficiency and Dysregulated Mitochondrial Function in Immortalized Lymphocytes from ME/CFS Patients. Int J Mol Sci. 2020 Feb 6;21(3):1074. doi: 10.3390/ijms21031074. PMID: 32041178; PMCID: PMC7036826.
- Behan WM, More IA, Behan PO. Mitochondrial abnormalities in the postviral fatigue syndrome. Acta Neuropathol. 1991;83(1):61-5. doi: 10.1007/BF00294431. PMID: 1792865.
- Sweetman E, Kleffmann T, Edgar C, de Lange M, Vallings R, Tate W. A SWATH-MS analysis of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome peripheral blood mononuclear cell proteomes reveals mitochondrial dysfunction. J Transl Med. 2020 Sep 24;18(1):365. doi: 10.1186/s12967-020-02533-3. PMID: 32972442; PMCID: PMC7512220.
- Schreiner P, Harrer T, Scheibenbogen C, Lamer S, Schlosser A, Naviaux RK, Prusty BK. Human Herpesvirus-6 Reactivation, Mitochondrial Fragmentation, and the Coordination of Antiviral and Metabolic Phenotypes in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Immunohorizons. 2020 Apr 23;4(4):201-215. doi: 10.4049/immunohorizons.2000006. PMID: 32327453.
- Missailidis D, Sanislav O, Allan CY, Smith PK, Annesley SJ, Fisher PR. Dysregulated Provision of Oxidisable Substrates to the Mitochondria in ME/CFS Lymphoblasts. Int J Mol Sci. 2021 Feb 19;22(4):2046. doi: 10.3390/ijms22042046. PMID: 33669532; PMCID: PMC7921983.
- Zhang C, Baumer A, Mackay IR, Linnane AW, Nagley P. Unusual pattern of mitochondrial DNA deletions in skeletal muscle of an adult human with chronic fatigue syndrome. Hum Mol Genet. 1995 Apr;4(4):751-4. doi: 10.1093/hmg/4.4.751. PMID: 7633428.
- Kennedy G, Spence V, Underwood C, Belch JJ. Increased neutrophil apoptosis in chronic fatigue syndrome. J Clin Pathol. 2004 Aug;57(8):891-3. doi: 10.1136/jcp.2003.015511. PMID: 15280416; PMCID: PMC1770396.
- Frémont M, D’Haese A, Roelens S, et al. Immune cell apoptosis and chronic fatigue syndrome. Chapter 6. In: Chronic Fatigue Syndrome. A Biological Approach. Englebienne P, De Meirleir K, editors, Boca Raton: CRC Press; 2002. p. 131 -74
- Nunes JM, Kruger A, Proal A, Kell DB, Pretorius E. The Occurrence of Hyperactivated Platelets and Fibrinaloid Microclots in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Pharmaceutical. 2022 July;15(8), 931; https://doi.org/10.3390/ph15080931
- Tomas C, Brown AE, Newton JL, Elson JL. Mitochondrial complex activity in permeabilised cells of chronic fatigue syndrome patients using two cell types. PeerJ. 2019 Mar 1;7:e6500. doi: 10.7717/peerj.6500. PMID: 30847260; PMCID: PMC6398432.

> some inborn errors of metabolism present in this manner, with an acute stress causing the body to suddenly lose the ability to compensate for the altered metabolite; though usually in childhood or adolescence.
This sounds a little like Robert Phair’s “bistability” hypothesis. Can you name some conditions which have this characteristic? I would be interested to read up more on them.
Hey Jason, here are two articles I found; one for medical professionals and one scientific publication:
ED Management of Inborn Errors of Metabolism
The liver is a metabolic and immunologic organ: a reconsideration of metabolic decompensation due to infection in inborn errors of metabolism (IEM)
Here is a quote from the second article: